Publications
2025
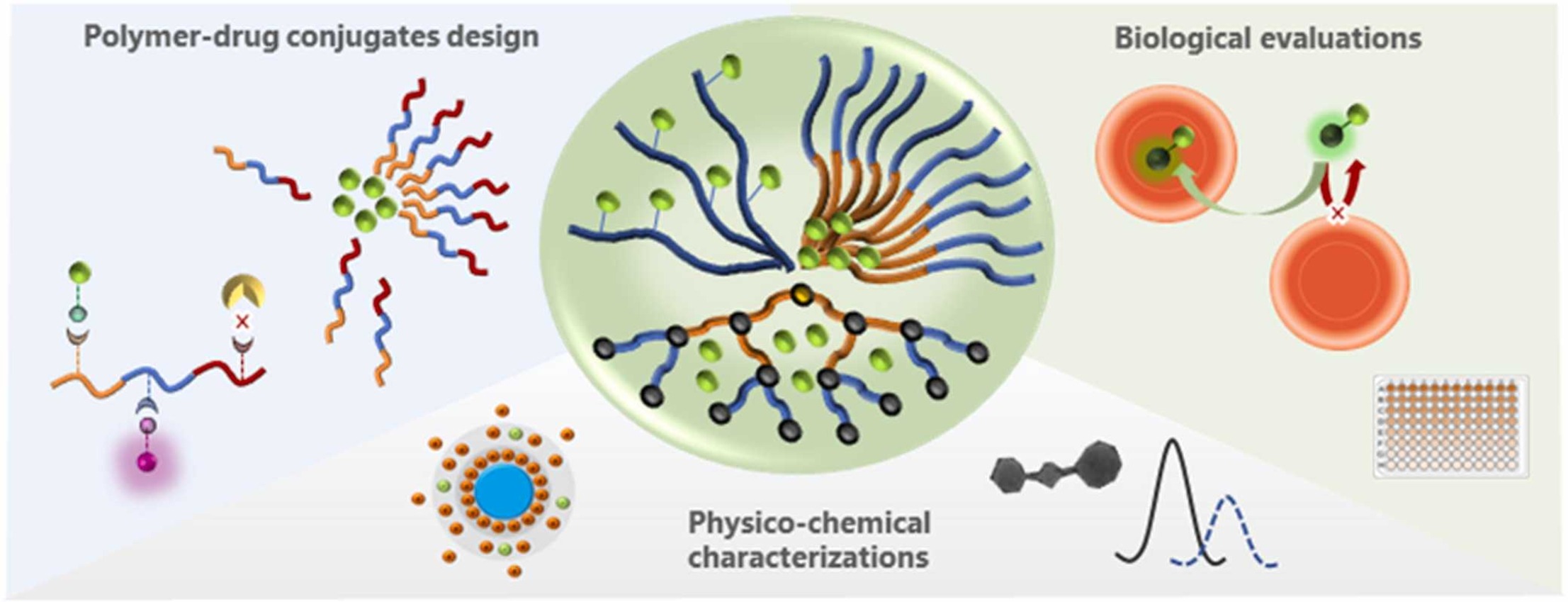
A how-to guide through the physico-chemistry and biology of polymeric drug delivery systems: Antimalarial vectors as study case 10.1016/j.nxmate.2024.100394
Casanova, Marion C.; Vanelle, Patrice; Azas, Nadine; Broggi, Julie Next Materials 2025, 6, 100394
2024
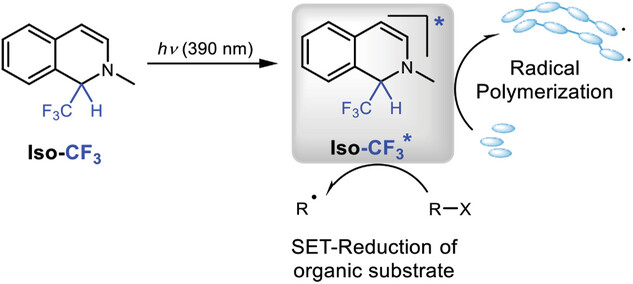
α-Trifluoromethylated quinolines as safe and storable PET-donor for radical polymerizations DOI: 10.1002/marc.202400710
Fall, Arona; Tintori, Guillaume; Rollet, Marion; Zhao, Yuxi; Avenel, Amandine; Charles, Laurence; Bergé-Lefranc, David; Clément, Jean Louis; Redon, Sébastien; Gigmes, Didier; Huix-Rotllant, Miquel; Vanelle, Patrice; Broggi, Julie, Macromol. Rapid Commun. 2024, 2400710
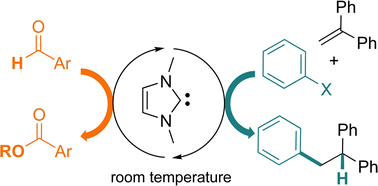
A Simple N-Heterocyclic Carbene for the Catalytic Up-conversion of Aldehydes into Stoichiometric Super Electron Donors 10.1039/D4SC04011B
Assani, Nadhrata; Delfau, Ludivine; Smits, Preslav; Redon, Sébastien; Kabri, Youssef; Tomás-Mendivil, Eder; Vanelle, Patrice; Martin, David; Broggi, Julie Chem. Sci. 2024, 15, 14699
2023
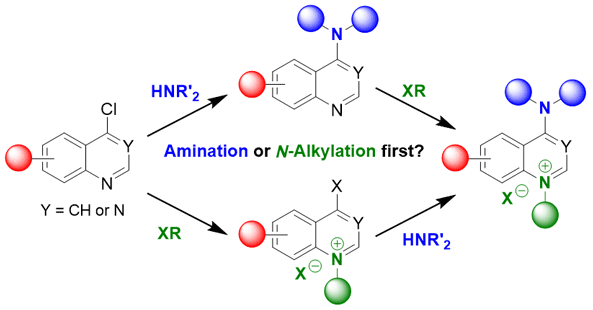
Efficient synthesis of amino-quinolinium and amino-quinazolinium salt series: Amination or N-Alkylation first? 10.1055/s-0042-1751465
Casanova, Marion C.; Fil, Maximilien; Zhao, Yuxi; Azas, Nadine; Redon, Sébastien; Vanelle, Patrice; Broggi, Julie Synlett 2023, 34, 1685-1688
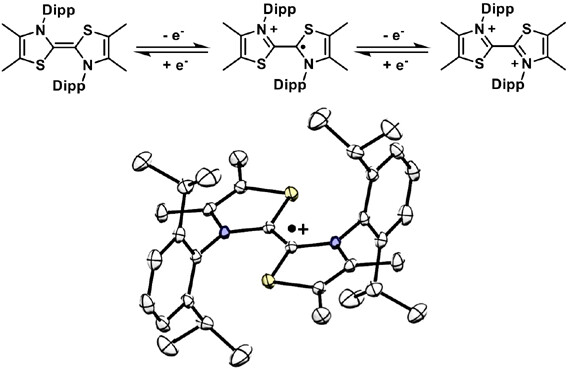
On the redox properties of the dimers of thiazol-2-ylidenes that are relevant for radical catalysis 10.1021/acsorginorgau.3c00008
Delfau, Ludivine; Assani, Nadhrata; Nichilo, Samantha; Pecaut, Jacques; Philouze, Christian; Broggi, Julie; Martin, David; Tomás-Mendivil, Eder ACS Org. Inorg. Au 2023, 3, 136-142
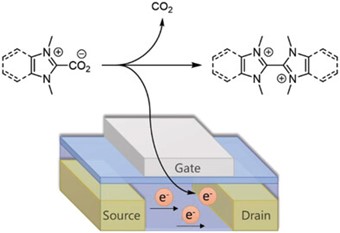
In situ generation of n-type dopants by thermal decarboxylation 10.1002/adfm.202212305
Aniés, Filip; Nugraha, Mohamad I.; Fall, Arona; Panidi, Julianna; Zhao, Yuxi; Vanelle, Patrice; Tsetseris, Leonidas; Broggi, Julie; Anthopoulos, Thomas D.; Heeney, Martin Adv. Funct. Mater. 2023, 2212305
2022
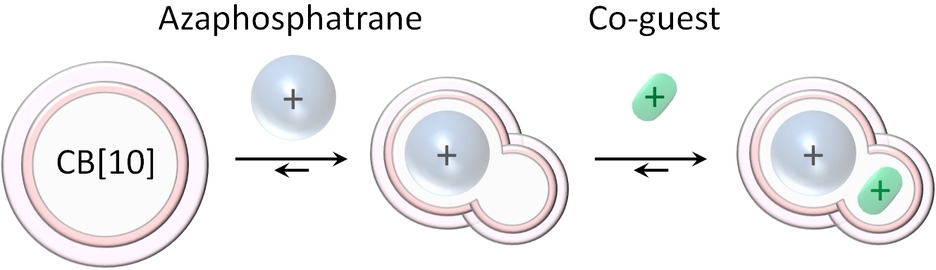
Sequential formation of heteroternary CB[10] complexes 10.1002/chem.202201656
Li, Chunyang; Manick, Anne-Doriane; Zhao, Yuxi; Liu, Fengbo; Chatelet, Bastien; Rosas, Roselyne; Siri, Didier; Gigmes, Didier; Monnier, Valerie; Charles, Laurence; Broggi, Julie; Liu, Simin; Martinez, Alexandre; Kermagoret, Anthony; Bardelang, David; Chem. Eur. J. 2022, 28, e202201656
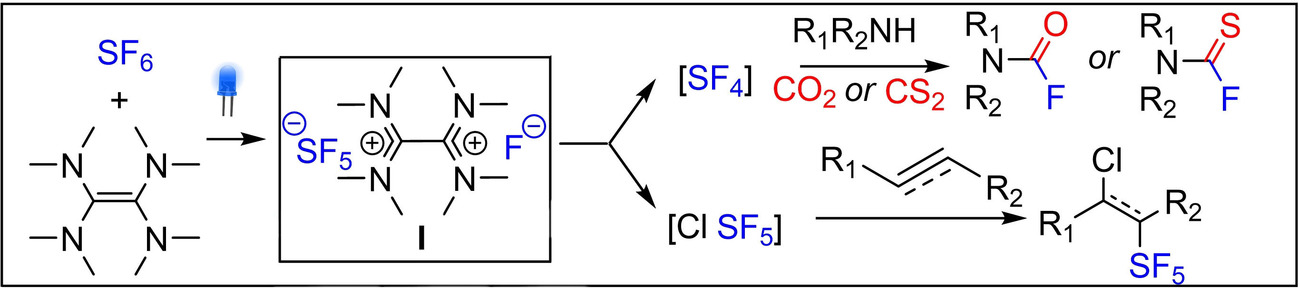
Metal-Free SF6 Activation: A New SF5-Based Reagent Enables Deoxyfluorination and Pentafluorosulfanylation Reactions 10.1002/anie.202204623
Taponard, Alexis; Jarrosson, Tristan; Khrouz, Lhoussain; Médebielle, Maurice; Broggi, Julie; Tlili, Anis; Angew. Chem. Int. Ed. 2022, 61, e202204623.
2021
Critical Assessment of the Reducing Ability of Breslow-type Derivatives and Implications for Carbene-catalyzed Radical Reactions 10.1002/anie.202111988
Delfau, Ludivine; Nichillo, Samantha; Molton, Florian; Broggi, Julie; Tomás-Mendivil, Eder; Martin, David Angew. Chem. Int. Ed. 2021, 60, 26783-26789
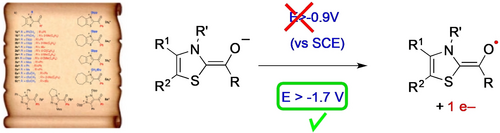
Switching from single to simultaneous free radical and anionic polymerization with enamine-based organic electron donors https://doi.org/10.1002/anie.202106733
Yuxi Zhao, Marion Rollet, Laurence Charles, Gabriel Canard, Didier Gigmes, Patrice Vanelle, Julie Broggi. Angew. Chem. Int. Ed., 2021, 60, 19389-19396
Beyond-Use Dates Assignment for Pharmaceutical Preparations: Example of Low-Dose Amiodarone Capsules https://journals.sagepub.com/doi/10.1177/87551225211015566
Brun, Damien; Curti, Christophe; Lamy, Edouard; Jean, Christophe; Bertault-Peres, Pierre; Broggi, Julie; Tintori, Guillaume; Vanelle, Patrice J. Pharm. Technol. 2021, 37, 178-185
Generation of powerful organic electron donors by water-assisted decarboxylation of benzimidazolinium carboxylates https://doi.org/10.1039/D0QO01488E
Guillaume Tintori, Arona Fall, Nadhrata Assani, Yuxi Zhao, David Bergé-lefranc, Sebastien Redon, Patrice Vanelle, Julie Broggi. J. Org. Org. Chem. Front., 2021, 8, 1197-1205
2020
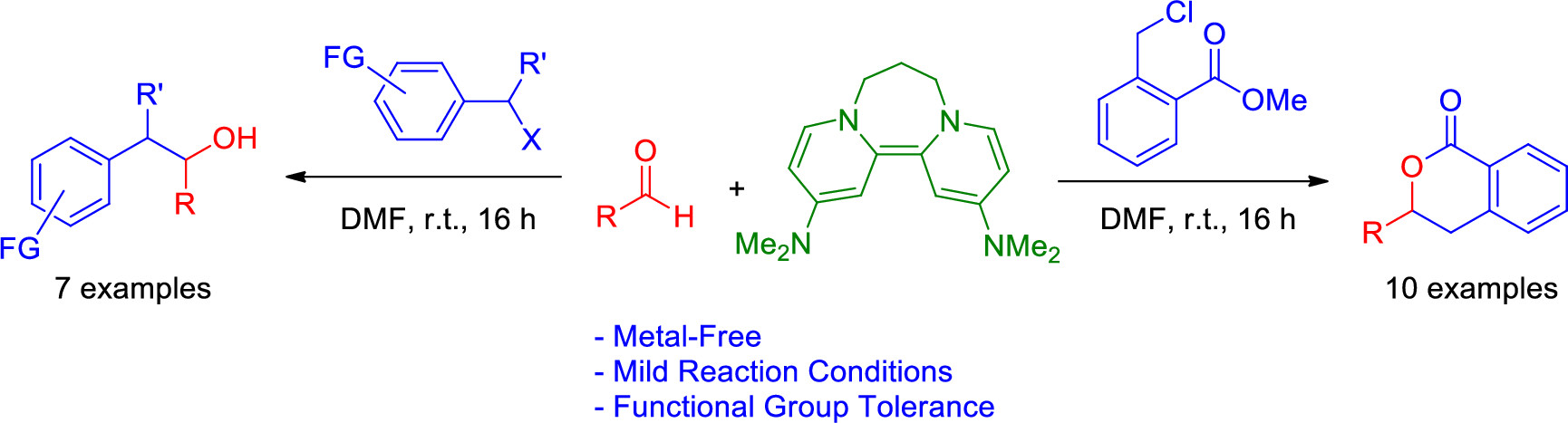
Metal-Free Addition of Benzyl Halides to Aldehyde using Super Electron Donor: Acess to 3,4-diDydoisocoumarins and 1,2-Diarylethanols https://dx.doi.org/10.1021/acs.joc.0c02374
Cédric Spitz, Mélanie Meutteudi, Guillaume Tintori, Julie Broggi, Thierry Terme and Patrice Vanelle. J. Org. Chem. 2020, 85, 15736-15742
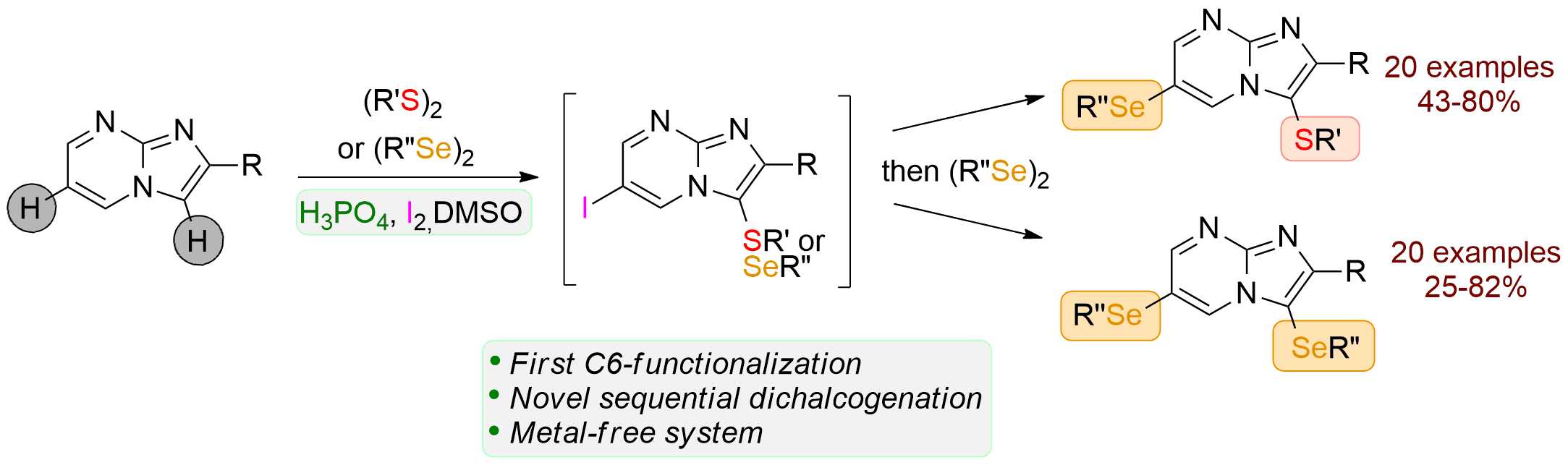
Sequential regioselective Diorganochalcogenations of Imidazo[1,2-a]pyrimidines Using I2/H3PO4 in Dimethylsulfoxide https://doi.org/10.1021/acs.joc.9b02963
Anne Roly Obah Kosso, Youssef Kabri, Julie Broggi, Sébastien Redon and Patrice Vanelle. J. Org. Chem. 2020
2019
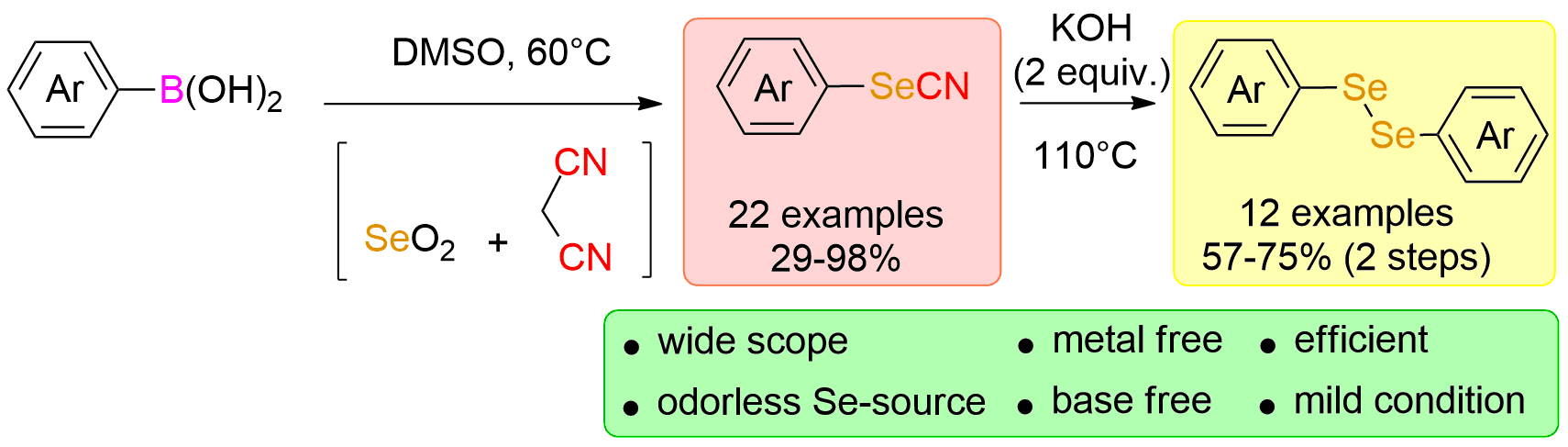
Metal-Free ispo-Selenocyanation of Arylboronic Acids Using Malononitrile and Selenium Dioxide https://doi.org/10.1055/s-0039-1690013
Anne Roly Obah Kosso, Julie Broggi, Sébastien Redon, Patrice Vanelle. Synthesis. 2019, 51, 3758-3764.
2018
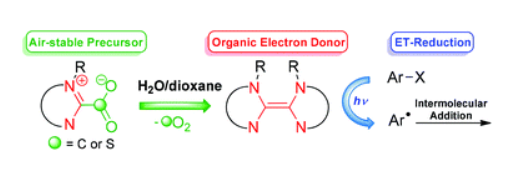
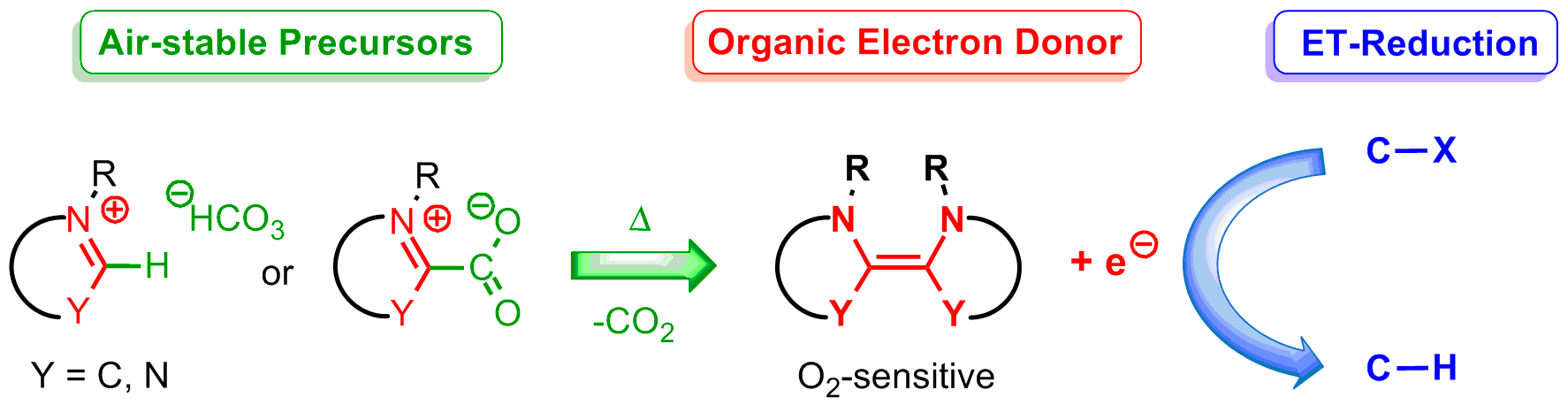
Base-free generation of organic electron donors from air-stable precursors https://doi.org/10.1002/anie.201713079
G. Tintori, P. Nabokoff, R. Buhaibeh, D. Bergé-Lefranc, S. Redon, J. Broggi, P. Vanelle. Angew. Chem. Int. Ed., 2018, 57, 3148-3153
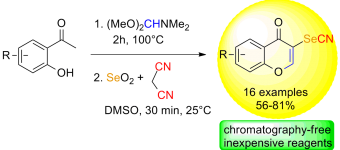
Convenient and rapid synthesis of 3-selenocyanato-4H-chromen-4-ones https://doi.org/10.1055/s-0037-1609340
A. R. Obah Kosso, J. Broggi, S. Redon, P. Vanelle. Synlett. 2018, 29, 1215-1218.
2017
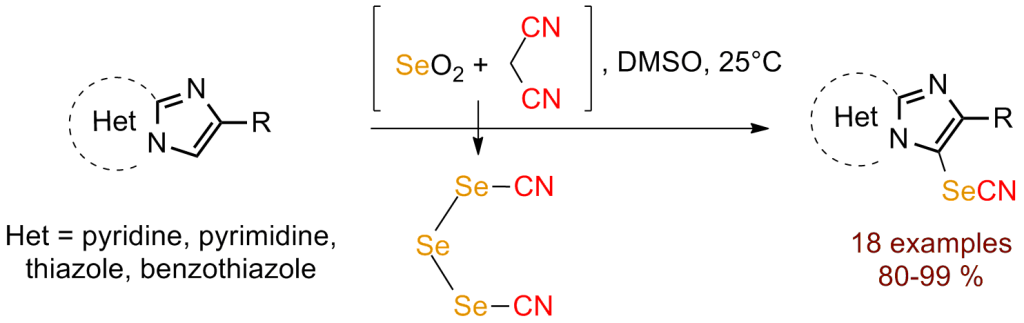
Easy and efficient selenocyanation of imidazoheterocycles using triselenodicyanide https://doi.org/10.1016/j.tetlet.2017.06.003
S. Redon, A. R. Obah Kosso, J. Broggi, P. Vanelle. Tetrahedron Lett. 2017, 58, 2771-2773.
2016
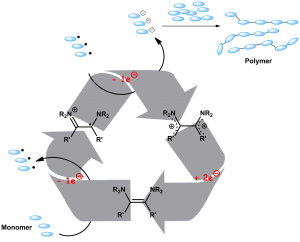
Polymerization Initiated by Organic Electron Donors https://doi.org/10.1002/anie.201600327
J. Broggi, M. Rollet, J.L. Clément, G. Canard, T. Terme, D. Gigmes, P. Vanelle. Angew. Chem. Int. Ed. 2016, 55, 5994-5999.
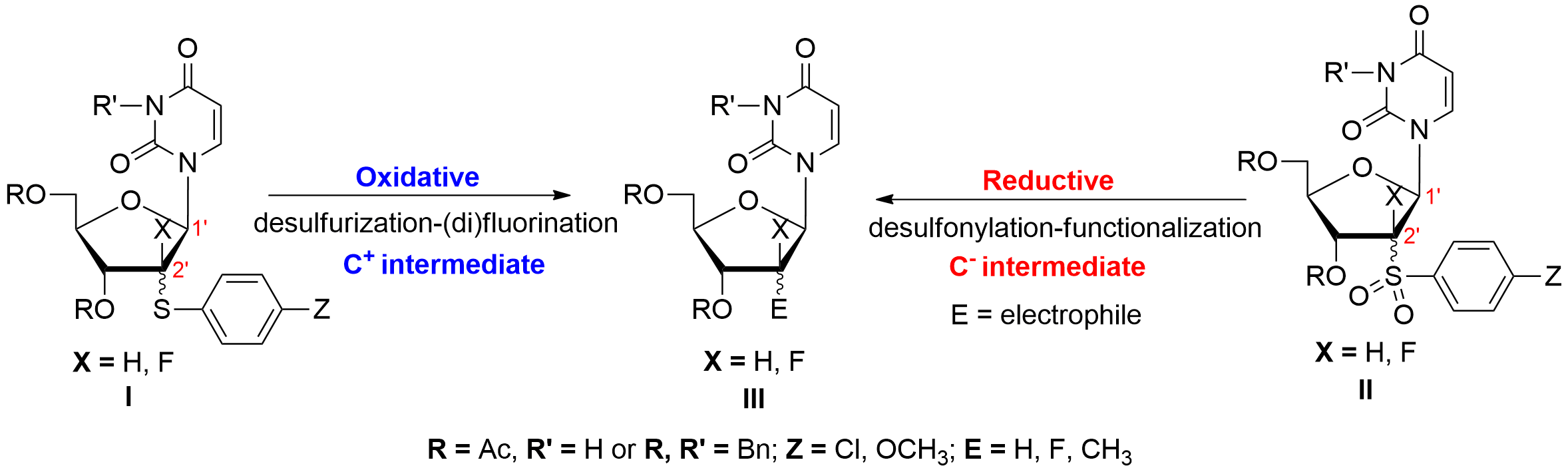
Studies toward the oxidative and reductive activation of C-S bonds in 2′-S-aryl-2′-thiouridine derivatives https://doi.org/10.1016/j.tet.2016.02.063
R. Rayala , A. Giuglio-Tonolo, J. Broggi, T. Terme, P. Vanelle, P. Theard, M. Médebielle, S. F. Wnuk. Tetrahedron, 2016, 72, 1969-1977.
2014
Organic Electron Donors as Powerful Single-Electron Transfer Reducing Agents in Organic Synthesis https://doi.org/10.1002/anie.201209060
J. Broggi, T. Terme, P. Vanelle, Angew. Chem., Int. Ed. 2014, 53, 384-413.
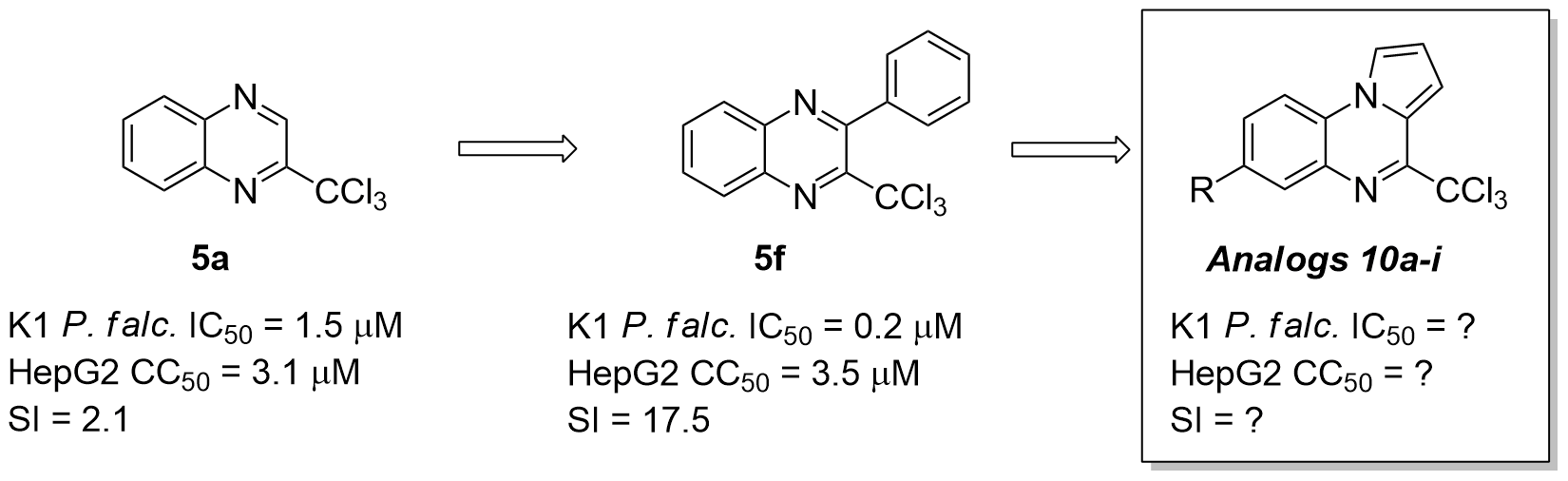
Synthesis and in vitro evaluation of 4-trichloromethylpyrrolo[1,2-a]quinoxalines as new antiplasmodial agents https://doi.org/10.1016/j.ejmech.2014.06.014
N. Primas, P. Suzanne, P. Verhaeghe, S. Hutter, C. Kieffer, M. Laget, A. Cohen, J. Broggi, , J. C. Lancelot, A. Lesnard, P. Dallemagne, P. Rathelot, S. Rault, P. Vanelle, N. Azas. Eur. J. Med. Chem. 2014, 83, 26-35.
2013
The Isolation of [Pd{OC(O)H}(H)(NHC)(PR3)] (NHC = N-Heterocyclic Carbene) and Its Role in Alkene and Alkyne Reductions Using Formic Acid https://doi.org/10.1021/ja311087c
J. Broggi, V. Jurčík, O. Songis, A. Poater, L. Cavallo, A. M. Z. Slawin, C. S. J. Cazin. J. Am. Chem. Soc. 2013, 135, 4588-4591.
2012
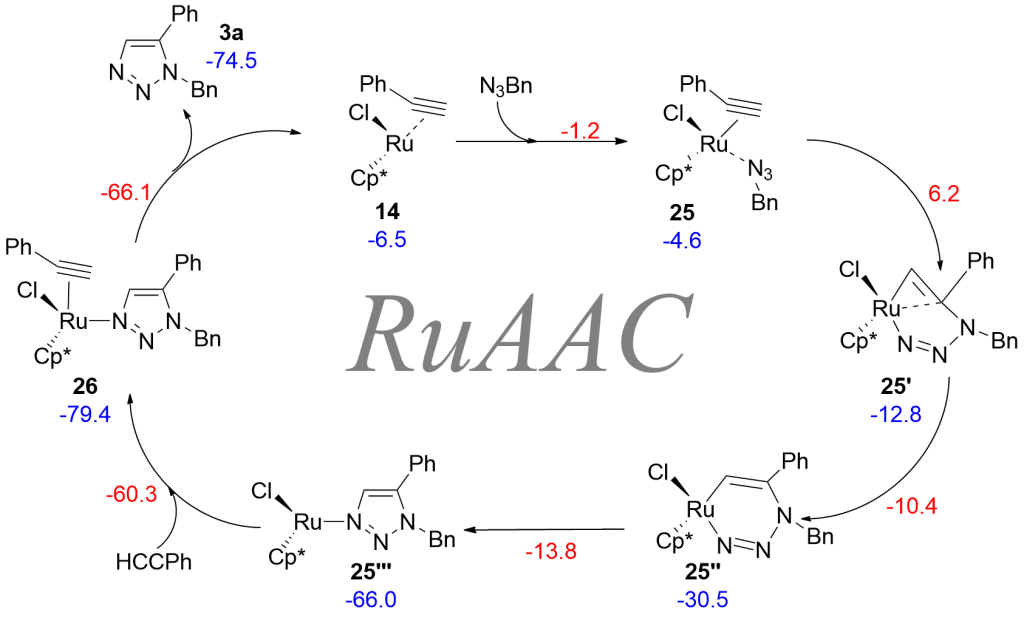
Coordinatively Unsaturated Ruthenium Complexes As Efficient Alkyne–Azide Cycloaddition Catalysts https://doi.org/10.1021/om2012425
M. Lamberti, G. C. Fortman, A. Poater, J. Broggi, A. M. Z. Slawin, L. Cavallo, S. P. Nolan. Organometallics, 2012, 31, 756-767.
2011
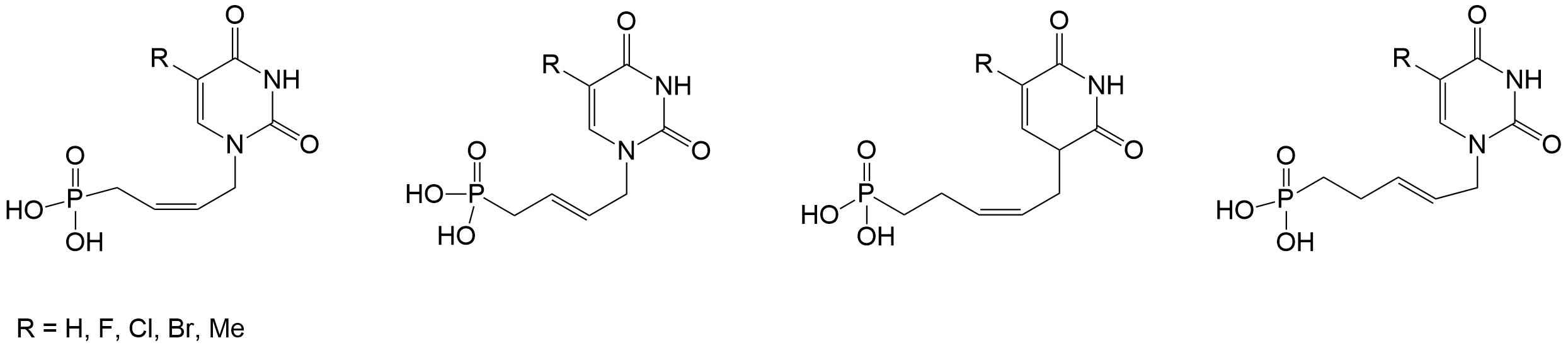
Novel Antiviral C5-Substituted Pyrimidine Acyclic Nucleoside Phosphonates Selected as Human Thymidylate Kinase Substrates https://doi.org/10.1021/jm1011462
D. Topalis, U. Pradère, V. Roy, C. Caillat, A. Azzouzi, J. Broggi, R. Snoeck, G. Andrei, J. Lin, S. Eriksson, J. A. C. Alexandre, C. El-Amri, D. Deville-Bonne, P. Meyer, J. Balzarini, L. A. Agrofoglio. J. Med. Chem. 2011, 54, 222-232.
2010
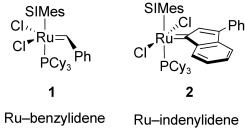
The Influence of Phosphane Ligands on the Versatility of Ruthenium-Indenylidene Complexes in Metathesis https://doi.org/10.1002/chem.201000659
J. Broggi, C. A. Urbina-Blanco, H. Clavier, A. Leitgeb, C. Slugovc, A. M.Z. Slawin, S. P. Nolan. Chem. Eur. J. 2010, 16, 9215-9225.
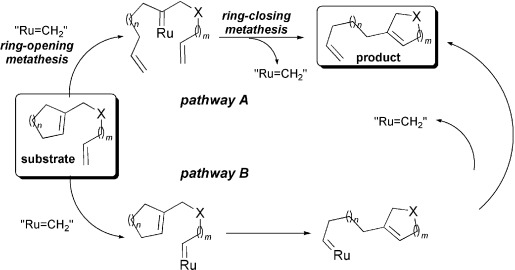
Ring-rearrangement metathesis (RRM) mediated by ruthenium-indenylidene complexes https://doi.org/10.1002/ejoc.200901316
H. Clavier, J. Broggi, S. P. Nolan. Eur. J. Org. Chem., 2010, 937-943.
2009
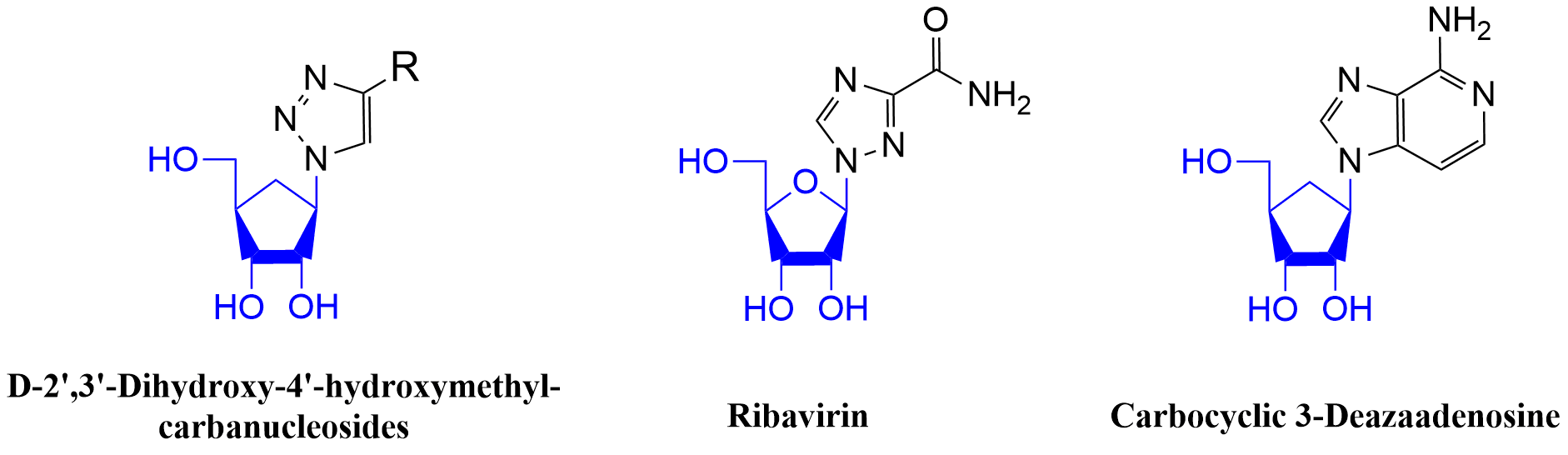
Click Azide-Alkyne Cycloaddition for the Synthesis of D-(-)-1,4-disubstituted-triazolo-carbanucleosides https://doi.org/10.1002/ejoc.200801124
J. Broggi, H. Kumamoto, S. Berteina-Raboin, S. P. Nolan, L. A. Agrofoglio. Eur. J. Org. Chem. 2009, 1880-1888.
Synthesis of (±)-1,2,3-triazolo-3’-deoxy-4’-hydroxymethyl carbanucleosides via ‘click’ cycloaddition https://doi.org/10.1016/j.tet.2008.11.065Get
Julie. Broggi,, Nicolas. Joubert, S. Díez-González, S. Berteina-Raboin, T. Zevaco, S. P. Nolan, L. A. Tetrahedron 2009, 65, 1162-1170.
2008
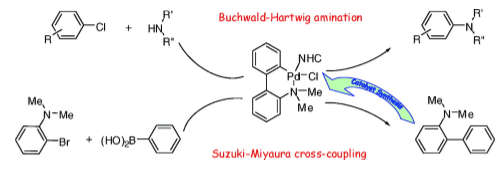
N-Heterocyclic Carbenes (NHCs) Containing N–C-Palladacycle Complexes: Synthesis and Reactivity in Aryl Amination Reactions https://doi.org/10.1021/om8006689
J. Broggi, H. Clavier, S. P. Nolan. Organometallics 2008, 27, 5525-5531.
Study of copper(I) catalysts for the synthesis of carbanucleosides via azide-alkyne 1,3-dipolar cycloaddition DOI: 10.1055/s-2007-990943
J. Broggi, S. Díez-González, J. L. Petersen, S. Berteina-Raboin, Steven P. Nolan, L. A. Agrofoglio. Synthesis, 2008, 141-148.
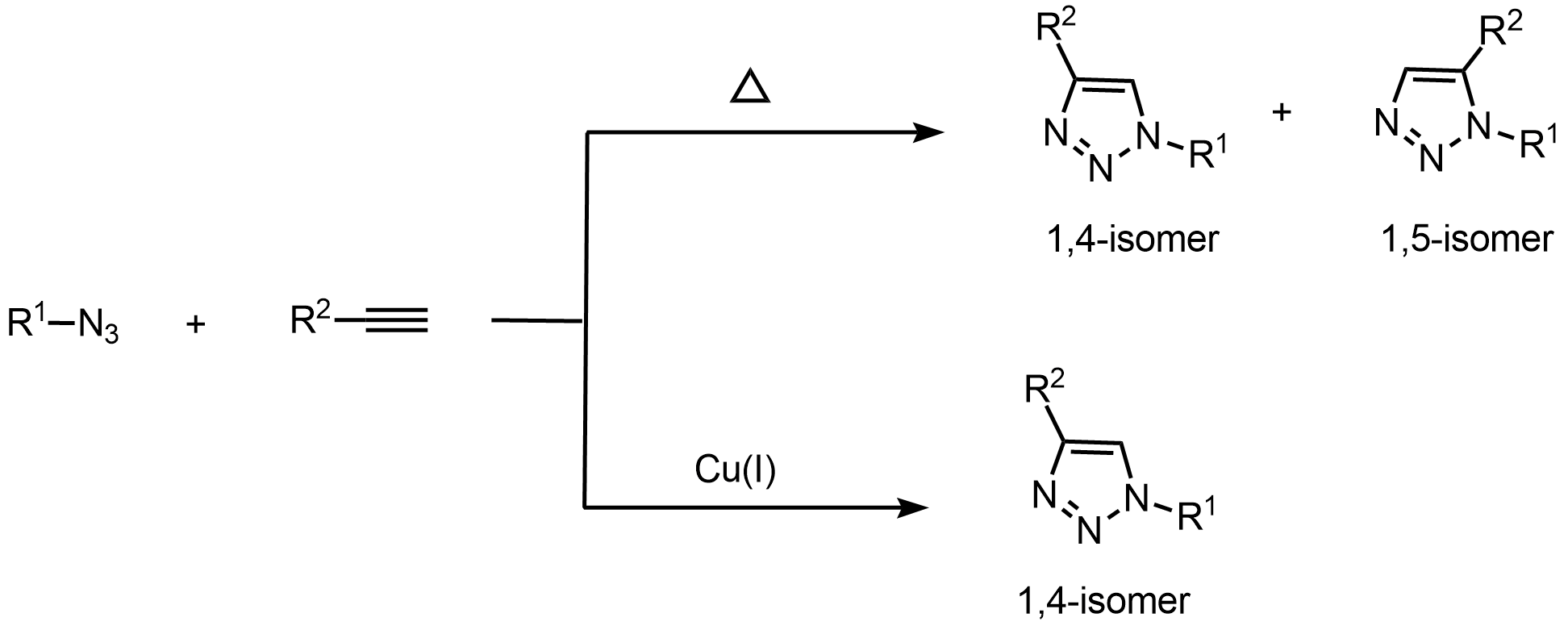
Preparation of acyclo nucleoside phosphonate analogues based on cross-metathesis https://doi.org/10.1021/om8006689
H. Kumamoto, D. Topalis, J. Broggi, U. Pradère, V. Roy, S. Berteina-Raboin, S. P. Nolan, D. Deville-Bonne, G. Andrei, R. Snoeck, D. Garin, J. M. Crance, L. A. Agrofoglio. Tetrahedron 2008, 64, 3517-3526.
2007
Study of Different Copper (I) Catalysts for the Click Chemistry Approach to Carbanucleosides https://doi.org/10.1080/15257770701501492
J. Broggi, N. Joubert, V. Aucagne, T. Zevaco, S. Berteina-Raboin, S.P. Nolan, L. A. Agrofoglio. Nucleosides, Nucleotides & Nucleic Acids, 2007, 26, 779-783
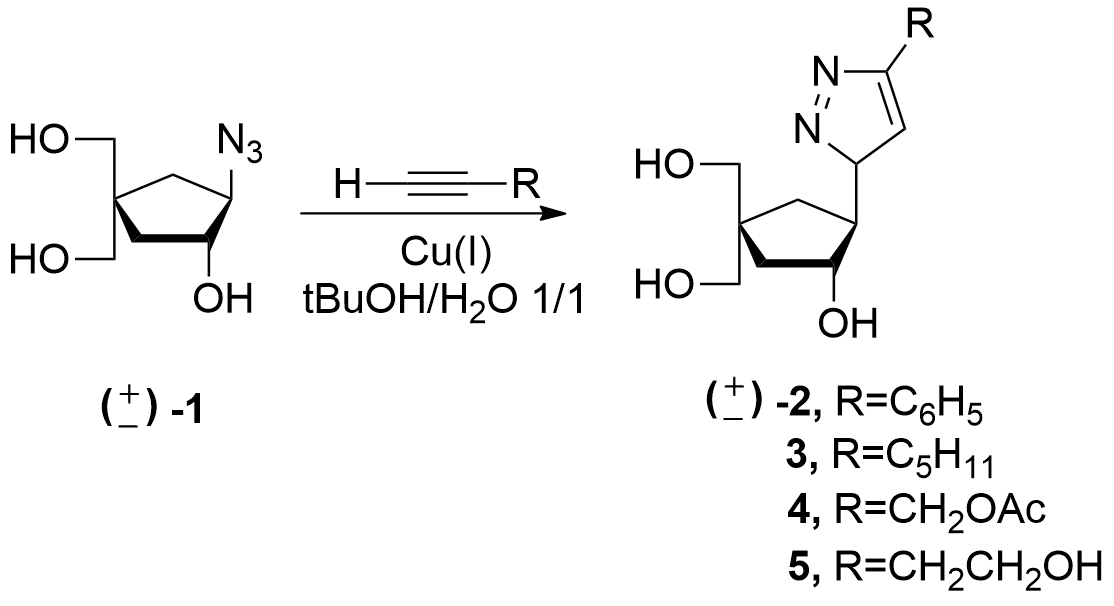
Alkyne-Azide Click Chemistry Mediated Carbanucleosides Synthesis https://doi.org/10.1080/15257770701534139
J. Broggi, N. Joubert, V. Aucagne, S. Berteina-Raboin, S. Diez-Gonzalez, S. Nolan, D. Topalis, D. Deville-Bonne, J. Balzarini, J.Neyts, G. Andrei, R. Snoeck, L. A. Agrofoglio. Nucleosides, Nucleotides & Nucleic Acids, 2007, 26, 1391-1394.